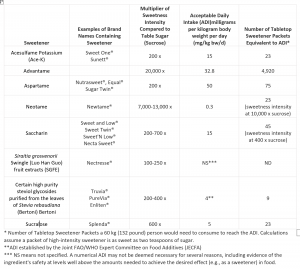
Exploring the facts and scientific evidence for some of the most common low-calorie sweeteners on the market
There’s probably no topic I get asked about more than artificial sweeteners: Are they safe? Do they enhance cravings? Do they cause weight gain? Do they affect the microbiome? I will admit that this was a topic I used to be very misinformed on, and although I personally don’t prefer the taste of most sugar substitutes, they certainly aren’t the poison I once believed they were.
I read a book in high school that talked about these high intensity sweeteners causing tumors in rats. That was enough to scare me because I didn’t understand how to evaluate the studies at the time. However, I now know that these rodent studies don’t have much relevance to the effects in humans at the amounts consumed. The doses given to the rats and mice in these studies are way above the amounts humans consume on any given day. Not to mention, the “sweet poison” claims aren’t based on human studies even though we do have quite a bit of data for most of these sugar substitutes. In fact, they are some of the most extensively researched ingredients in our food supply. So, let’s look at the facts and the evidence for some of the most common low calorie sweeteners on the market.
Sucralose
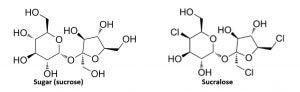
Sucralose is a no-calorie sweetener made from sugar (sucrose). Three select hydrogen-oxygen (hydroxyl) groups on a sucrose molecule are replaced with three chlorine atoms, resulting in a no-calorie sweetener that is about 600 times sweeter than sugar.
About 85 percent of consumed sucralose is not absorbed. Of the about 15 percent absorbed, none is broken down for energy, so sucralose does not provide any calories. All absorbed sucralose is excreted quickly in the urine.
More than 100 safety studies representing over 20 years of research have shown sucralose to be safe. Leading global health authorities such as the European Food Safety Authority, the FAO/WHO Joint Expert Committee on Food Additives, Japan’s Ministry of Health, Labor and Welfare, Food Standards Australia New Zealand, and Health Canada have also found sucralose to be safe.
The FDA established an acceptable daily intake (ADI) for sucralose of 5 milligrams per kilogram of body weight (mg/kg) per day. The ADI represents an amount 100 times less than the quantity of sucralose found to be safe in research studies. A 2018 scientific review found that studies conducted since 2008 raise no concerns for exceeding the ADI of the major low- and no-calorie sweeteners, including sucralose, in the general population.
Data from randomized controlled trials support that substituting low-calorie sweetener options for regular-calorie versions leads to modest weight loss. Extensive research shows that sucralose does not raise blood sugar levels or otherwise affect blood glucose control in humans, and a recent consensus statement by experts in nutrition, medicine, physical activity and public health concluded that the use of low-calorie sweeteners in diabetes management may contribute to better glycemic control.
To date there is no strong evidence that low-calorie sweeteners, including sucralose, enhance appetite or cravings in humans, and some randomized trials have demonstrated the opposite effect — including a decrease in hunger and reduced dessert intake compared to those who drank water. Others have shown no effect of sucralose on hormones that regulate hunger and fullness or on total energy intake and selection of sweet foods.
To date there are few studies on sucralose’s effect on the human gut microbiome, though it is known that it is not metabolized by the gut microbiota. In rodents, exposure to sucralose has resulted in wide-ranging, inconsistent effects. A 2019 literature review found no conclusive evidence that low-calorie sweeteners negatively impact gut microbiota. In 2020, a panel of experts on low-calorie sweeteners came to a similar conclusion that, at this time, data on the effects of low-calorie sweeteners on the human gut microbiota are limited and do not provide adequate evidence that they impact gut health at doses that are relevant to human consumption.
Aspartame
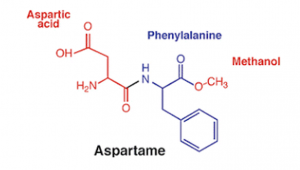
Aspartame is made up of two amino acids — aspartic acid and phenylalanine and is about 200 times sweeter than sugar (sucrose). Aspartame contains four calories per gram just like sugar, but since it’s so much sweeter than sugar, very little is needed to provide the same sweetness. Aspartame is broken down into aspartic acid and phenylalanine for use in protein synthesis and metabolism when ingested. A small amount of methanol (a compound naturally found in foods like fruits and vegetables and their juices) is also produced during digestion. Aspartame intake should be limited by people with phenylketonuria (PKU), which is a rare genetic disease that makes an affected person unable to properly metabolize phenylalanine.
Aspartame is one of the most extensively studied ingredients in our food supply, with more than 200 studies supporting its safety. Leading global health authorities such as the European Food Safety Authority and the FAO/WHO Joint Expert Committee on Food Additives conduct scientific risk assessments and safety evaluations of food additives and have concluded that aspartame is safe for its intended uses. Based on these conclusions and other independent reviews, government regulators around the world, including Japan’s Ministry of Health, Labor, and Welfare; Food Standards Australia New Zealand; Health Canada; and the U.S. Food and Drug Administration permit the use of aspartame.
The FDA has established an acceptable daily intake (ADI) for aspartame of 50 milligrams per kilogram of body weight (mg/kg) per day. The European Food Safety Authority has established a slightly lower ADI of 40 mg/kg per day. The ADI represents an amount 100 times less than the quantity of aspartame found to achieve a no-observed-adverse-effect-level (NOAEL). Globally, aspartame intake also remains well below the FDA and EFSA ADIs. A 2018 study noted that only in rare instances did individuals exceed more than 20 percent of the ADI, even in the highest-consuming groups.
Extensive research shows that aspartame does not raise blood glucose levels or otherwise affect blood glucose management in humans. In a 2018 randomized controlled trial, aspartame ingestion had no effect on blood glucose or insulin levels over the 12-week intervention as compared with a placebo.
Saccharin
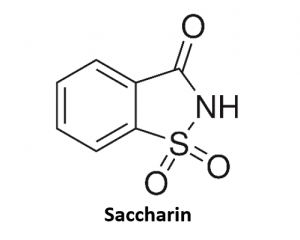
Saccharin is another no calorie sweetener since it isn’t broken down in our bodies and passes through the body unchanged, thus, provides no calories in the process. Saccharin is about 200 to 700 times sweeter than sugar (sucrose) and has been used to sweeten foods and beverages since 1900, making it the oldest low- or no-calorie sweetener approved for use by the FDA.
Saccharin is one of eight low and no-calorie sweeteners permitted by the FDA for use in the U.S. food supply. All eight have been rigorously tested and reviewed. Leading global health authorities such as the European Food Safety Authority, the FAO/WHO Joint Expert Committee on Food Additives, U.S. Food and Drug Administration, Food Standards Australia New Zealand, and Health Canada have found low and no-calorie sweeteners to be safe.
The FDA has established an acceptable daily intake (ADI) for saccharin of 15 milligrams per kilogram of body weight (mg/kg) per day. Intakes by high consumers of saccharin (90th percentile or higher) are orders of magnitude lower than the amounts used in many animal studies and are well below the saccharin ADI established by the JECFA and the Scientific Committee for Food.
Saccharin is safe to consume for individuals with diabetes. A recent study showed that high-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans.
“By studying the artificial sweetener saccharin in healthy adults, we’ve isolated its effects and found no change in participants’ gut microbiome or their metabolic profiles, as it was previously suggested,” said George Kyriazis, assistant professor of biological chemistry and pharmacology at Ohio State and senior author of the study.
Stevia
Stevia sweeteners are no calorie sweeteners derived from the leaves of the Stevia rebaudiana (Bertoni) plant, which is an herbal shrub that is native to South America. These sweeteners are 200 to 350 times sweeter than sugar (sucrose). Purified steviol glycosides do not contribute to any calories to our diet as they are not absorbed in the upper gastrointestinal tract. When they reach the colon, gut microbes cleave off the glucose molecules and use them as an energy source. The remaining steviol backbone is then absorbed via the portal vein, metabolized by the liver, and excreted in urine.
High-purity steviol glycosides were granted generally recognized as safe (GRAS) status from the FDA in 2008. However, whole stevia leaf and crude stevia extracts do not have FDA approval for use in foods or drinks. Leading global health authorities such as the European Food Safety Authority, the FAO/WHO Joint Expert Committee on Food Additives, the Japan Ministry of Health and Welfare, Food Standards Australia New Zealand, and Health Canada have also found purified stevia sweeteners to be safe.
The Joint Expert Committee on Food Additives established an acceptable daily intake (ADI) for stevia sweeteners of 4 milligrams of steviol equivalents per kilogram of body weight (mg/kg) per day. Globally, intake of stevia sweeteners remains well below the ADI. A 2018 scientific review found that studies conducted since 2008 raise no concerns for exceeding the ADI of the major low- and no-calorie sweeteners, including stevia sweeteners, in the general population.
Studies have shown no effect of stevia sweeteners on satiety and a reduction in overall daily energy intake compared to a full-sugar control, which is attributed to the lower calorie content of the stevia sweetener intervention and the fact that participants did not make up for the deficit by eating more calories later in the day. Additionally, a recent study demonstrated a reduction in hunger after consuming cookies made with stevia sweeteners compared to control cookies.
Extensive research shows that stevia sweeteners do not raise blood sugar levels in humans, and a recent consensus statement by experts in nutrition, medicine, physical activity, and public health concluded that the use of low-calorie sweeteners in diabetes management may contribute to better glycemic control.
Even though the gut microbiota are integral to the metabolism of steviol glycosides, to date there is no evidence that stevia sweeteners meaningfully impact the composition or function of the gut microbiome. However, randomized clinical trials have not yet been conducted in humans.
Monk fruit
Monk fruit, also known as lo han guo or Swingle fruit (Siraitia grosvenorii), is a small round fruit native to southern China. Monk fruit sweeteners are created by removing the seeds and skin of the fruit, crushing the fruit, and collecting the juice. The fruit extract, or juice, contains zero calories per serving. Monk fruit sweeteners are 150 to 200 times sweeter than sugar (sucrose).
Mogrosides are the compounds that give ripe monk fruit its sweetness. They consist of a backbone structure called a mogrol with glucose units or glycosides attached to it. Most of what is known about mogroside metabolism comes from animal studies, which is thought to be the same or similar to mogroside metabolism in humans. Mogrosides do not contribute to any calories to our diet as they are not absorbed in the upper gastrointestinal tract. When they reach the colon, gut microbes cleave off the glucose molecules and use them as an energy source. The mogrol and some metabolites are then primarily excreted from the gastrointestinal tract, while minor amounts are absorbed into the bloodstream and excreted in the urine.
Monk fruit sweeteners were granted generally recognized as safe (GRAS) status from the FDA in 2010. Governments in Australia and New Zealand, China, Japan, and Canada (tabletop packets only; not approved for use in foods and beverages) have also concluded that monk fruit sweeteners are safe for the general population. An acceptable daily intake (ADI) has not been established for monk fruit sweeteners because adverse effects have not been demonstrated, even after high amounts of monk fruit sweeteners were given in animal studies.
No research has been published on the specific effects of monk fruit sweeteners on appetite and satiety, however, one small study demonstrated that total daily energy intake did not differ between people consuming monk fruit sweeteners and those consuming sugar.
Research has shown that monk fruit sweeteners do not raise blood sugar levels in humans, and a recent consensus statement by experts in nutrition, medicine, physical activity and public health concluded that the use of low-calorie sweeteners in diabetes management may contribute to better glycemic control.
Even though the gut microbiota are integral to the metabolism of monk fruit’s mogrosides, to date there is no evidence that monk fruit sweeteners meaningfully impact the composition or function of the gut microbiome. However, randomized clinical trials have not yet been conducted in humans.
In addition to the FDA approved high intensity sweeteners discussed above, there are a few additional ones, including acesulfame potassium (Ace-K), neotame and advantame that you can learn more about at the links provided as well as here.
So, what’s the bottom line on low calorie sweeteners? These compounds are some of the most extensively studied ingredients in our food supply. They can be a great alternative to sugar for those with diabetes and can be helpful in weight loss and weight maintenance. They are a safe option for anyone who is looking for ways to reduce calories, carbohydrates, and added sugars without sacrificing sweet taste.
Food Science Babe is the pseudonym of an agvocate and writer who focuses specifically on the science behind our food. She has a degree in chemical engineering and has worked in the food industry for more than decade, both in the conventional and in the natural/organic sectors.



